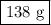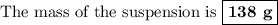Chemistry, 21.08.2019 20:30 jescanarias22
Ca + 2h2o --> ca(oh)2 + h2
40g of ca is mixed with 100g of water. calculate mass of solution left after reaction is complete.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

Chemistry, 23.06.2019 01:00
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1
You know the right answer?
Ca + 2h2o --> ca(oh)2 + h2
40g of ca is mixed with 100g of water. calculate mass of soluti...
40g of ca is mixed with 100g of water. calculate mass of soluti...
Questions



Mathematics, 15.10.2019 02:00


Chemistry, 15.10.2019 02:00

Mathematics, 15.10.2019 02:00


History, 15.10.2019 02:00

Mathematics, 15.10.2019 02:00


Mathematics, 15.10.2019 02:00

Biology, 15.10.2019 02:00


Mathematics, 15.10.2019 02:00

Mathematics, 15.10.2019 02:00