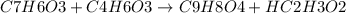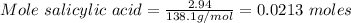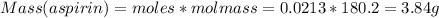Chemistry, 21.08.2019 22:30 hooplikeapro
What is the theoretical yield of aspirin (180.2 g/mol) in the reaction of 2.94 grams of salicylic acid (138.1 g/mol) with excess acetic anhydride (102.1 g/mol)? show your work. include units and round your final answer appropriately.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2


Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
You know the right answer?
What is the theoretical yield of aspirin (180.2 g/mol) in the reaction of 2.94 grams of salicylic ac...
Questions

English, 05.05.2021 23:00

Mathematics, 05.05.2021 23:00


Chemistry, 05.05.2021 23:00

Mathematics, 05.05.2021 23:00


French, 05.05.2021 23:00

Mathematics, 05.05.2021 23:00

Social Studies, 05.05.2021 23:00



English, 05.05.2021 23:00

Mathematics, 05.05.2021 23:00


Mathematics, 05.05.2021 23:00



Mathematics, 05.05.2021 23:00

Business, 05.05.2021 23:00

Mathematics, 05.05.2021 23:00