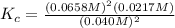Chemistry, 22.08.2019 01:10 AmityHeart
Consider the following reaction: 2nobr(g) 2no(g) + br2(g)if 0.412 moles of nobr(g), 0.678 moles of no, and 0.224 moles of br2 are at equilibrium in a 10.3 l container at 516 k, the value of the equilibrium constant, kc, is:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
Consider the following reaction: 2nobr(g) 2no(g) + br2(g)if 0.412 moles of nobr(g), 0.678 moles of n...
Questions


Arts, 27.08.2019 09:00

Mathematics, 27.08.2019 09:00



Physics, 27.08.2019 09:00

History, 27.08.2019 09:00

Mathematics, 27.08.2019 09:00

Health, 27.08.2019 09:00


Computers and Technology, 27.08.2019 09:00


Mathematics, 27.08.2019 09:00

History, 27.08.2019 09:00

Social Studies, 27.08.2019 09:00

Mathematics, 27.08.2019 09:00

History, 27.08.2019 09:00

Mathematics, 27.08.2019 09:00


Mathematics, 27.08.2019 09:00

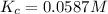
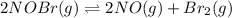
![K_c=\frac{[products]}{[reactants]}](/tpl/images/0186/4128/cdede.png)
![K_c=\frac{[NO]^2[Br_2]}{[NOBr]^2}](/tpl/images/0186/4128/967c8.png)
![[NOBr]=\frac{0.412mol}{10.3L}](/tpl/images/0186/4128/ea500.png) = 0.040 M
= 0.040 M![[NO]=\frac{0.678mol}{10.3L}](/tpl/images/0186/4128/0105d.png) = 0.0658 M
= 0.0658 M![[Br_2]=\frac{0.224mol}{10.3L}](/tpl/images/0186/4128/3e384.png) = 0.0217 M
= 0.0217 M