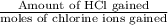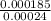Chemistry, 22.08.2019 03:30 shymitch32
Ahittorf cell fitted with ag/agcl electrodes is filled with 0.0106 molal aqueous hcl (molar mass: 36.458). a 2.00 ma current was passed for 10800 sec. the cathode solution was then found to weigh 51.7436 g and contain 0.0267 g hcl after analysis. what is the transport number of h+?
a. 0.177
b. 0355
c. 0.645
d. 0.823

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
Ahittorf cell fitted with ag/agcl electrodes is filled with 0.0106 molal aqueous hcl (molar mass: 3...
Questions




















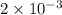 A
A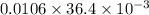
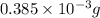
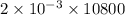 = 21.6 C
= 21.6 C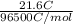
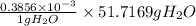
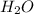 = 0.0267 g
= 0.0267 g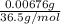
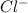 in cathodic compartment = 0.000224 mol
in cathodic compartment = 0.000224 mol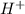 =
=