
Chemistry, 22.08.2019 16:10 achsahjosey
An experiment is carried out to produce hydrogen gas. hydrochloric acid is poured into a glass, and magnesium is added to the acid. the reaction produces a soluble salt and hydrogen gas. 2.1 write a balanced equation for the reaction. 2.2 give the name of the soluble salt. 2.3 has a physical or chemical change taken place? 2.4 2 moles of hydrogen is produced. how many moles of acid reacted?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3
You know the right answer?
An experiment is carried out to produce hydrogen gas. hydrochloric acid is poured into a glass, and...
Questions



Computers and Technology, 25.03.2021 21:10

Biology, 25.03.2021 21:10



History, 25.03.2021 21:10

Mathematics, 25.03.2021 21:10




Mathematics, 25.03.2021 21:10


Business, 25.03.2021 21:10



Mathematics, 25.03.2021 21:10

Arts, 25.03.2021 21:10


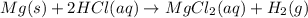
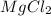
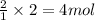 of HCl
of HCl

