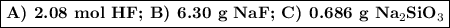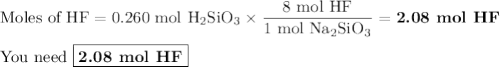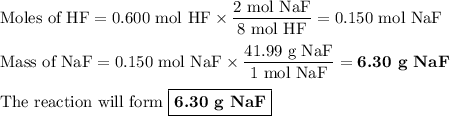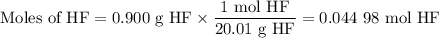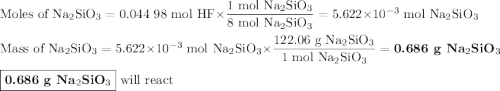Hydrofluoric acid, hf(aq), cannot be stored in glass bottles because compounds called silicates in the glass are attacked by the hf(aq). sodium silicate (na2sio3), for example, reacts as follows: na2sio3(s)+8hf(aq)→h2sif6(aq)+2naf( aq)+3h2o(l)? a)how many moles of hf are needed to react with 0.260 mol of na2sio3? b) how many grams of naf form when 0.600 mol of hf reacts with excess na2sio3? c)how many grams of na2sio3 can react with 0.900 g of hf?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
Hydrofluoric acid, hf(aq), cannot be stored in glass bottles because compounds called silicates in t...
Questions


Mathematics, 14.01.2021 19:30


Mathematics, 14.01.2021 19:30

Mathematics, 14.01.2021 19:30

Advanced Placement (AP), 14.01.2021 19:30

Physics, 14.01.2021 19:30

Mathematics, 14.01.2021 19:40


Spanish, 14.01.2021 19:40



Mathematics, 14.01.2021 19:40


History, 14.01.2021 19:40



Biology, 14.01.2021 19:40