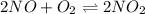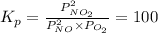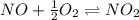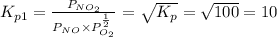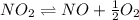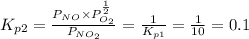Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Consider the balanced equation below. n2h4 + 2h2o2 n2 + 4h2o what are the mole ratios of hydrazine (n2h4) to hydrogen peroxide (h2o2) and hydrazine to water? 1: 2 and 1: 4 1: 3 and 1: 4 1: 2 and 3: 5 1: 3 and 3: 5
Answers: 3

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
Find the equilibrium constants, kp, for the following equilibria, (i) no(g) + ½ o2(g) ⇄ no2(g), kp =...
Questions

Mathematics, 07.05.2021 20:10

Mathematics, 07.05.2021 20:10

Engineering, 07.05.2021 20:10



History, 07.05.2021 20:10




Mathematics, 07.05.2021 20:10

Mathematics, 07.05.2021 20:10


Mathematics, 07.05.2021 20:10

English, 07.05.2021 20:10

Chemistry, 07.05.2021 20:10

Mathematics, 07.05.2021 20:10

History, 07.05.2021 20:10

Chemistry, 07.05.2021 20:10


Mathematics, 07.05.2021 20:10