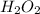Chemistry, 22.08.2019 18:10 ggdvj9gggsc
Given the reaction: h2o2(l) ⇌ h2(g) + o2(g) the forward reaction is endothermic. determine which of the following changes would result in equilibrium shifting towards the products. i. increase h2 ii. decrease o2 iii. add a catalyst iv. increase the temperature v. increase h2o2

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
Given the reaction: h2o2(l) ⇌ h2(g) + o2(g) the forward reaction is endothermic. determine which of...
Questions


Computers and Technology, 29.07.2019 08:00





Computers and Technology, 29.07.2019 08:00

Computers and Technology, 29.07.2019 08:00


Physics, 29.07.2019 08:00

Mathematics, 29.07.2019 08:00





History, 29.07.2019 08:00

Computers and Technology, 29.07.2019 08:00


History, 29.07.2019 08:00

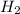 and
and 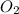 and reactant is
and reactant is