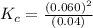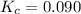Chemistry, 22.08.2019 18:30 bjpvrpow74wq
At high temperatures, carbon reacts with o2 to produce co as follows: c(s) o2(g) 2co(g). when 0.350 mol of o2 and excess carbon were placed in a 5.00-l container and heated, the equilibrium concentration of co was found to be 0.060 m. what is the equilibrium constant, kc, for this reaction?
a. 0.001
b. 0.072
c. 0.090
d. 1.2

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
At high temperatures, carbon reacts with o2 to produce co as follows: c(s) o2(g) 2co(g). when 0.350...
Questions



Physics, 21.04.2021 02:00

Advanced Placement (AP), 21.04.2021 02:00





Geography, 21.04.2021 02:00


Chemistry, 21.04.2021 02:00

Mathematics, 21.04.2021 02:00




Mathematics, 21.04.2021 02:00


Mathematics, 21.04.2021 02:00


Computers and Technology, 21.04.2021 02:00

 = 0.350 mole
= 0.350 mole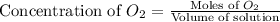
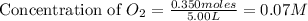
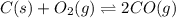
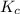 will be,
will be,![K_c=\frac{[CO]^2}{[O_2]}](/tpl/images/0188/5908/f4b67.png)
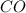 at equilibrium is, 0.060 M
at equilibrium is, 0.060 M