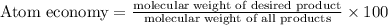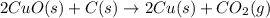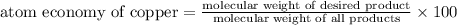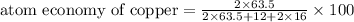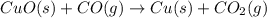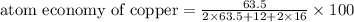Chemistry, 22.08.2019 19:10 GEEKLIFE6598
The following two reactions are possible methods for refining copper in the final step of a smelting process, i. e., getting pure copper (cu) from copper ores found in rocks. calculate the theoretical atom economy for each reaction. a. 2 cuo(s) + c(s) → 2 cu(s) + co2(g) = % b. cuo(s) + co(g) → cu(s) + co2(g) = %

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3
You know the right answer?
The following two reactions are possible methods for refining copper in the final step of a smelting...
Questions

English, 23.02.2021 16:10



Mathematics, 23.02.2021 16:10






Mathematics, 23.02.2021 16:10



Social Studies, 23.02.2021 16:10


World Languages, 23.02.2021 16:10

Biology, 23.02.2021 16:10