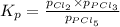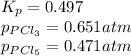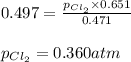Chemistry, 22.08.2019 19:20 wafflewarriormg
The equilibrium constant, kp, for the following reaction is 0.497 at 500k. pcl5(g) pcl3(g) + cl2(g)if an equilibrium mixture of the three gases in a 18.4 l container at 500k contains pcl5 at a pressure of 0.471 atm and pcl3 at a pressure of 0.651 atm, the equilibrium partial pressure of cl2 is atm.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
The equilibrium constant, kp, for the following reaction is 0.497 at 500k. pcl5(g) pcl3(g) + cl2(g)i...
Questions

Mathematics, 29.07.2019 05:10

Mathematics, 29.07.2019 05:10

Mathematics, 29.07.2019 05:10

Mathematics, 29.07.2019 05:10

Social Studies, 29.07.2019 05:10





Biology, 29.07.2019 05:10

English, 29.07.2019 05:10



Mathematics, 29.07.2019 05:10


Business, 29.07.2019 05:10

Business, 29.07.2019 05:10



English, 29.07.2019 05:10

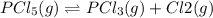
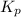 for above reaction follows:
for above reaction follows: