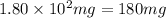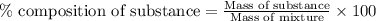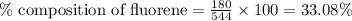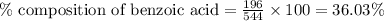A544 mg of a mixture of fluorene and benzoic acid was weighed out and subjected to an extraction and recrystallization. after this purification was completed the product crystals were dried and analyzed. the purification procedure produced 1.80 × 102 mg of fluorene and 196 mg of benzoic acid. calculate the percent composition of this mixture.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
A544 mg of a mixture of fluorene and benzoic acid was weighed out and subjected to an extraction and...
Questions

Chemistry, 10.07.2019 14:40


History, 10.07.2019 14:40


Mathematics, 10.07.2019 14:40


Social Studies, 10.07.2019 14:40

Social Studies, 10.07.2019 14:40

Mathematics, 10.07.2019 14:40

Social Studies, 10.07.2019 14:40



History, 10.07.2019 14:40

Mathematics, 10.07.2019 14:40





Social Studies, 10.07.2019 14:40