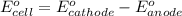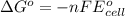Chemistry, 22.08.2019 21:30 trosclairozlynn02
Using the following standard reduction potentials, fe3+(aq) + e- → fe2+(aq) e° = +0.77 v ni2+(aq) + 2 e- → ni(s) e° = -0.23 v calculate the standard cell potential for the galvanic cell reaction given below, and determine whether or not this reaction is spontaneous under standard conditions. ni2+(aq) + 2 fe2+(aq) → 2 fe3+(aq) + ni(s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

You know the right answer?
Using the following standard reduction potentials, fe3+(aq) + e- → fe2+(aq) e° = +0.77 v ni2+(aq) +...
Questions

Mathematics, 24.10.2020 19:10


Mathematics, 24.10.2020 19:10

Chemistry, 24.10.2020 19:10


Social Studies, 24.10.2020 19:10

Mathematics, 24.10.2020 19:10


Mathematics, 24.10.2020 19:10

Biology, 24.10.2020 19:10

Health, 24.10.2020 19:10



Mathematics, 24.10.2020 19:20


Mathematics, 24.10.2020 19:20

Mathematics, 24.10.2020 19:20

Mathematics, 24.10.2020 19:20


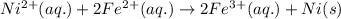
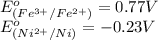
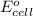 of the reaction, we use the equation:
of the reaction, we use the equation: