
Chemistry, 22.08.2019 22:30 emmaraeschool
The equilibrium constant kc for the reaction pcl3(g) + cl2(g) ⇌ pcl5(g) is 49 at 230°c. if 0.70 mol of pcl3 is added to 0.70 mol of cl2 in a 1.00-l reaction vessel at 230°c, what is the concentration of pcl3 when equilibrium has been established? a) 0.049 mb) 0.11 mc) 0.59 md) 0.30 me) 0.83 m

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
The equilibrium constant kc for the reaction pcl3(g) + cl2(g) ⇌ pcl5(g) is 49 at 230°c. if 0.70 mol...
Questions

English, 03.07.2019 22:30




History, 03.07.2019 22:30


Arts, 03.07.2019 22:30

Mathematics, 03.07.2019 22:30


Mathematics, 03.07.2019 22:30



Arts, 03.07.2019 22:30


Biology, 03.07.2019 22:30


English, 03.07.2019 22:30

History, 03.07.2019 22:30

Mathematics, 03.07.2019 22:30

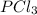 and
and 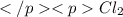 .
.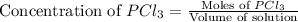
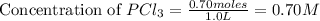
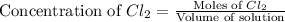
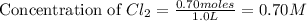
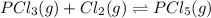
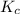 will be,
will be,![K_c=\frac{[PCl_5]}{[PCl_3][Cl_2]}](/tpl/images/0189/1195/4c8d0.png)
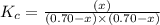
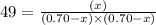
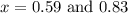
 at equilibrium = (0.70-x) = (0.70-0.59) = 0.11 M
at equilibrium = (0.70-x) = (0.70-0.59) = 0.11 M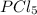 at equilibrium = x = 0.59 M
at equilibrium = x = 0.59 M

