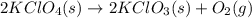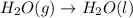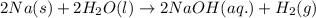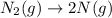Chemistry, 23.08.2019 01:30 hallmansean04
4. without consulting appendix 3 in the textbook, predict whether the entropy change is positive or negative for each of the following reactions. give reasons for your predictions. (12 points) a. 2kclo4(s) → 2kclo3(s) + o2(g) b. h2o(g) → h2o(l) c. 2na(s) + 2h2o(l) → 2naoh(aq) + h2(g) d. n2(g) → 2n(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
4. without consulting appendix 3 in the textbook, predict whether the entropy change is positive or...
Questions




















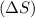 . Randomness of gaseous particles is more than that of liquid which is further more than that of solids.
. Randomness of gaseous particles is more than that of liquid which is further more than that of solids.