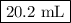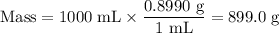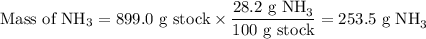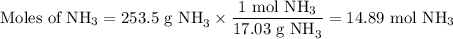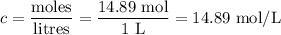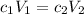Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1


Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
Astock solution is 28.2 percent ammonia (nh3) by mass, and the solution has a density of 0.8990 gram...
Questions


Mathematics, 31.07.2019 22:00





History, 31.07.2019 22:00

Mathematics, 31.07.2019 22:00



Computers and Technology, 31.07.2019 22:00


Social Studies, 31.07.2019 22:00

Computers and Technology, 31.07.2019 22:00

Computers and Technology, 31.07.2019 22:00

Computers and Technology, 31.07.2019 22:00


Computers and Technology, 31.07.2019 22:00