
Calcium reacts with bromine to form calcium bromide. 2.1 which atom will lose electrons? 2.2 how many electrons will it lose? 2.3 name the element that will gain electrons. 2.4 how many electrons does this atom gain? 2.5 write down the symbol for each of the ions formed. 2.6 write the chemical formula for calcium bromide.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

You know the right answer?
Calcium reacts with bromine to form calcium bromide. 2.1 which atom will lose electrons? 2.2 how ma...
Questions



Mathematics, 11.02.2021 19:00




Mathematics, 11.02.2021 19:00



Social Studies, 11.02.2021 19:00

Mathematics, 11.02.2021 19:00



Geography, 11.02.2021 19:00

Chemistry, 11.02.2021 19:00


Mathematics, 11.02.2021 19:00


Mathematics, 11.02.2021 19:00

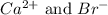
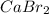
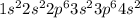
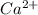 ion.
ion.![[Ar]4s^23d^{10}4p^5](/tpl/images/0191/9443/d71de.png)
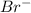 ion.
ion.

