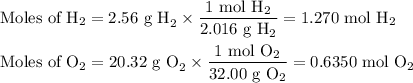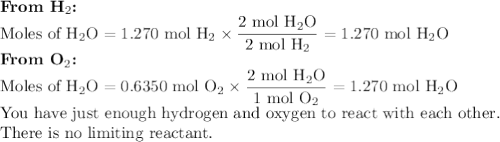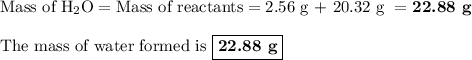Chemistry, 25.08.2019 19:30 tae8002001
Hydrogen and oxygen undergo a chemical reaction to form water. how much water would form if 2.56 g of hydrogen reacted completely with 20.32 g of oxygen? g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2
You know the right answer?
Hydrogen and oxygen undergo a chemical reaction to form water. how much water would form if 2.56 g o...
Questions

Biology, 27.05.2021 01:00

Computers and Technology, 27.05.2021 01:00

English, 27.05.2021 01:00




Arts, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00



Social Studies, 27.05.2021 01:00


Business, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00


Mathematics, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00