***30
if 25.0 ml of a 0.100 m aqueous sodium hydroxide is mixed with 25.0 ml of a 0.100 m aqu...

Chemistry, 25.08.2019 21:10 Andrebutrus
***30
if 25.0 ml of a 0.100 m aqueous sodium hydroxide is mixed with 25.0 ml of a 0.100 m aqueous hydrochloric acid in a calorimeter at an initial temperature of 23.0 degrees celsius, what is the enthalpy change of this reaction if the final temperature reached in the calorimeter is 25.5 degrees celsius?
naoh + hcl yields nacl + h2o

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
Questions

History, 01.07.2019 19:30


Biology, 01.07.2019 19:30


Mathematics, 01.07.2019 19:30





Mathematics, 01.07.2019 19:30





History, 01.07.2019 19:30

History, 01.07.2019 19:30

History, 01.07.2019 19:30



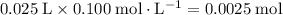 of both NaOH and HCl are available. As a result, 0.0025 moles of the reaction would have taken place.
of both NaOH and HCl are available. As a result, 0.0025 moles of the reaction would have taken place.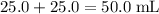 .
.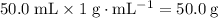 .
.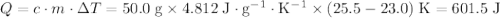
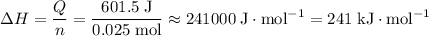 .
.

