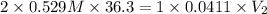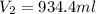Chemistry, 26.08.2019 16:10 nightangel175
A36.3 ml aliquot (sample) of 0.529 m h2so4 (aq) is to be titrated with 0.0411 m naoh. what volume of base will it take to reach the equivalence point?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1
You know the right answer?
A36.3 ml aliquot (sample) of 0.529 m h2so4 (aq) is to be titrated with 0.0411 m naoh. what volume of...
Questions

Mathematics, 01.03.2021 04:00

English, 01.03.2021 04:00

Arts, 01.03.2021 04:00

Mathematics, 01.03.2021 04:00





World Languages, 01.03.2021 04:00

Mathematics, 01.03.2021 04:00







Mathematics, 01.03.2021 04:00

Mathematics, 01.03.2021 04:00

Mathematics, 01.03.2021 04:00

Social Studies, 01.03.2021 04:00

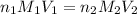
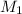 = molarity of
= molarity of 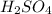 solution = 0.529 M
solution = 0.529 M
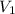 = volume of
= volume of 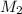 = molarity of
= molarity of 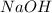 solution = 0.0411 M
solution = 0.0411 M
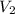 = volume of
= volume of 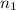 = valency of
= valency of 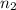 = valency of
= valency of