
Chemistry, 26.08.2019 18:00 juan01sebastian00
Nitrogen (n2) and hydrogen (h2) react to form ammonia (nh3). consider a mixture of six nitrogen molecules and six hydrogen molecules in a closed container. assuming the reaction goes to completion, what will the final product mixture be?
a. number of nh3 molecules
b. number of n2 molecules
c. number of h2 molecules
which of the following equations best represents this reaction?
a. 42 n2 + 6 h2 4 nh3
b. 6 n2 + 6 h2 4 nh3 + 4 n2
c. n + 3 h2 nh3
d. n2 + 3 h2 2 nh3
e. n2 + h2 nh3

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
Nitrogen (n2) and hydrogen (h2) react to form ammonia (nh3). consider a mixture of six nitrogen mole...
Questions


History, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00

History, 02.03.2021 06:00

Social Studies, 02.03.2021 06:00

Social Studies, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00






Mathematics, 02.03.2021 06:00

Chemistry, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00




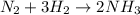
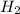 react completely with 1 molecule of
react completely with 1 molecule of 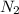 and produce 2 molecules of
and produce 2 molecules of 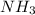 .
.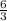 or 2 molecules of
or 2 molecules of  or 4 molecules of
or 4 molecules of 

