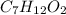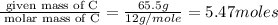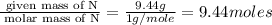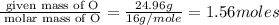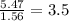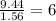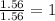Chemistry, 26.08.2019 20:10 baileyedavis
An unknown compound contains only the three elements c, h, and o. a pure sample of the compound is analyzed and found to be 65.60 percent c and 9.44 percent h by mass. (a) determine the empirical formula of the compound.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1


Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
An unknown compound contains only the three elements c, h, and o. a pure sample of the compound is a...
Questions

Computers and Technology, 20.01.2021 20:00

Chemistry, 20.01.2021 20:00

Mathematics, 20.01.2021 20:00

Mathematics, 20.01.2021 20:00

History, 20.01.2021 20:00

Mathematics, 20.01.2021 20:00


Business, 20.01.2021 20:00

Mathematics, 20.01.2021 20:00



Mathematics, 20.01.2021 20:00

Arts, 20.01.2021 20:00

Mathematics, 20.01.2021 20:00



Mathematics, 20.01.2021 20:00

Mathematics, 20.01.2021 20:00

Biology, 20.01.2021 20:00

Mathematics, 20.01.2021 20:00