
Chemistry, 27.08.2019 00:10 addisonrausch
Butane c4h10 ( = –125.7), combusts in the presence of oxygen to form co2 (g) (hf = –393.5 kj/mol), and h2o(g) (hf = –241.82) in the reaction:
2c4h10 (g) + 13o2 (g) = 8co2 (g) + 10h2o (g)
what is the enthalpy of combustion, per mole, of butane? (a) -2,657.5 kj/mol(b) -5315.0 kj/mol(c) -509.7 kj/mol(d) -254.8 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2
You know the right answer?
Butane c4h10 ( = –125.7), combusts in the presence of oxygen to form co2 (g) (hf = –393.5 kj/mol), a...
Questions





History, 02.10.2019 21:40

Business, 02.10.2019 21:40

English, 02.10.2019 21:40

Biology, 02.10.2019 21:40



Mathematics, 02.10.2019 21:40

Mathematics, 02.10.2019 21:40


Mathematics, 02.10.2019 21:40

Physics, 02.10.2019 21:40



English, 02.10.2019 21:40

Computers and Technology, 02.10.2019 21:40


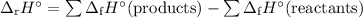
![\begin{array}{rcl}\Delta_{\text{c}}H^{\circ} & = & [8(-393.5) + 10(-241.82)] - 2(-125.7)\\& = & [-3148.0 - 2418.2] +251.4\\& = & -5566.2 + 251.4\\& = & -5314.8\\\end{array}\\\\\text{This is the value for 2 mol of butane.}\\\text{The enthalpy of combustion is } \dfrac{-5314.8}{2} = \boxed{\textbf{-2657.4 kJ/mol}}](/tpl/images/0200/8107/1b095.png)



