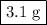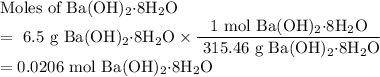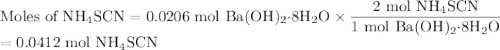Chemistry, 27.08.2019 00:20 insaneshootermo
One of relatively few reactions that takes place directly between two solids at room temperature is ba(oh)2 · 8h2o + ammonium thiocyanate --> barium thiocyanate + water + ammonia in this equation, the · 8h2o in ba(oh)2 · 8h2o indicates the presence of eight water molecules. this compound is called barium hydroxide octahydrate. what mass of ammonium thiocyanate must be used if it is to react completely with 6.5 g barium hydroxide octahydrate?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3


Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
One of relatively few reactions that takes place directly between two solids at room temperature is...
Questions




Mathematics, 18.10.2020 01:01

History, 18.10.2020 01:01

History, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01


English, 18.10.2020 01:01


Mathematics, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01

English, 18.10.2020 01:01

Biology, 18.10.2020 01:01





Mathematics, 18.10.2020 01:01