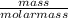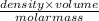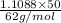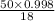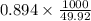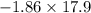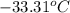Chemistry, 27.08.2019 02:30 marissa2367
The coolant in automobiles is often a 50/50 % by volume mixture of ethylene glycol, c2h6o2, and water. at 20°c, the density of ethylene glycol is 1.1088 g/ml and the density of water is 0.9982 g/ml. what is the expected freezing point of a 50/50(v/v)% ethylene glycol/water solution? kf = 1.86°c/m for water.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
The coolant in automobiles is often a 50/50 % by volume mixture of ethylene glycol, c2h6o2, and wate...
Questions


Mathematics, 13.11.2019 22:31

History, 13.11.2019 22:31




Physics, 13.11.2019 22:31

Chemistry, 13.11.2019 22:31