
Chemistry, 27.08.2019 02:30 camballard3848
The freezing point of ethanol (c2h5oh) is -114.6 °c. the molal freezing point depression constant for ethanol is 2.00 °c/m. what is the freezing point (°c) of a solution prepared by dissolving 50.0 g of glycerin (c3h8o3, a nonelectrolyte) in 200 g of ethanol

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
The freezing point of ethanol (c2h5oh) is -114.6 °c. the molal freezing point depression constant fo...
Questions

Mathematics, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00




Mathematics, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

Advanced Placement (AP), 18.03.2021 03:00


Biology, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00

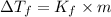
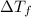 = Depression in freezing point
= Depression in freezing point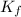 = Molal freezing point depression constant
= Molal freezing point depression constant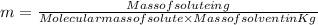
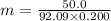
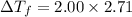 = 5.42 °C
= 5.42 °C

