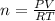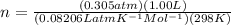Chemistry, 27.08.2019 16:20 TheSmartRey
Asolution of 62.4 g of insulin in enough water to make 1.000 l of solution has an osmotic pressure of 0.305 atm at 25°c. based on these data, what is the molar mass of insulin?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

You know the right answer?
Asolution of 62.4 g of insulin in enough water to make 1.000 l of solution has an osmotic pressure o...
Questions

English, 28.02.2021 03:20


Spanish, 28.02.2021 03:20

Mathematics, 28.02.2021 03:20

Health, 28.02.2021 03:20


English, 28.02.2021 03:20

History, 28.02.2021 03:20

Mathematics, 28.02.2021 03:20

English, 28.02.2021 03:20


Mathematics, 28.02.2021 03:20

Mathematics, 28.02.2021 03:20





Mathematics, 28.02.2021 03:20

English, 28.02.2021 03:20