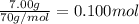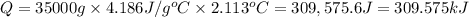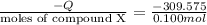Chemistry, 27.08.2019 17:10 sakurauchiha913
7.00g of compound x with molecular formula c5h10 are burned in a constant-pressure calorimeter containing 35.00kg of water at 25°c. the temperature of the water is observed to rise by 2.113°c. (you may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) calculate the standard heat of formation of compound x at 25°c. be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. [could you show a step by step way to solve this]

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
If a 60-g object has a volume of 30 cm3, what is its density? 2 g/cm3 0.5 cm3/g 1800 g * cm3 none of the above
Answers: 3

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1


Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
7.00g of compound x with molecular formula c5h10 are burned in a constant-pressure calorimeter conta...
Questions

Social Studies, 11.11.2019 09:31

Chemistry, 11.11.2019 09:31


Mathematics, 11.11.2019 09:31

Biology, 11.11.2019 09:31






Mathematics, 11.11.2019 09:31


History, 11.11.2019 09:31



History, 11.11.2019 09:31



History, 11.11.2019 09:31

History, 11.11.2019 09:31