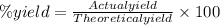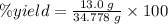Chemistry, 27.08.2019 17:10 hopelesslylost13
In making wine, glucose (c6h12o6) is fermented to produce ethanol (c2h5oh) and carbon dioxide (co2), according to the following reaction. c6h12o6 → 2 c2h5oh + 2 co2 (a) if the fermentation reaction starts with 68.0 g glucose, what is the theoretical yield of ethanol (in grams)? g (b) if 13.0 g ethanol is produced, what is the percent yield of this reaction?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1
You know the right answer?
In making wine, glucose (c6h12o6) is fermented to produce ethanol (c2h5oh) and carbon dioxide (co2),...
Questions





Chemistry, 18.09.2019 00:00




Computers and Technology, 18.09.2019 00:00


Computers and Technology, 18.09.2019 00:00







English, 18.09.2019 00:00

Computers and Technology, 18.09.2019 00:00