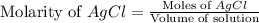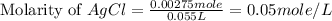Suppose 0.701g of iron(ii) chloride is dissolved in 50.ml of a 55.0mm aqueous solution of silver nitrate.
calculate the final molarity of chloride anion in the solution. you can assume the volume of the solution doesn't change when the iron(ii) chloride is dissolved in it.
be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
Suppose 0.701g of iron(ii) chloride is dissolved in 50.ml of a 55.0mm aqueous solution of silver nit...
Questions










Computers and Technology, 20.12.2019 20:31










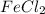 = 0.701 g
= 0.701 g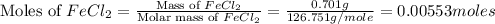
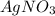 .
.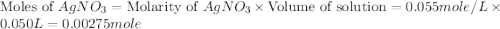
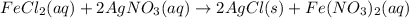
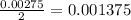 moles of
moles of 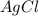 .
.