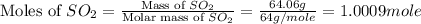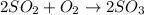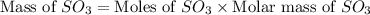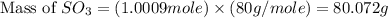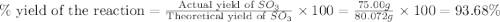In the manufacturing process of sulfuric acid, sulfur dioxide is reacted with oxygen to produce sulfur trioxide. using the equation, 2so2 (g) + o2 imported asset 2so3 (g), if 64.06g of sulfur dioxide is given an opportunity to react with an excess of oxygen to produce 75.00 g of sulfur trioxide, what is the percent yield of this reaction? 46.83% 60.25% 75.55% 93.68%

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2


Chemistry, 23.06.2019 10:30
Silver is a white metal that is an excellent conductor. silver tarnishes when exposed to air and light. the density of silver is 10.49 g/cm3. the melting point is 962oc and the boiling point is 2000oc. a chemical property of silver is
Answers: 3
You know the right answer?
In the manufacturing process of sulfuric acid, sulfur dioxide is reacted with oxygen to produce sulf...
Questions

Chemistry, 23.10.2020 03:01


Chemistry, 23.10.2020 03:01

Biology, 23.10.2020 03:01

History, 23.10.2020 03:01

Geography, 23.10.2020 03:01

Advanced Placement (AP), 23.10.2020 03:01

History, 23.10.2020 03:01

Arts, 23.10.2020 03:01


Health, 23.10.2020 03:01


Mathematics, 23.10.2020 03:01





Computers and Technology, 23.10.2020 03:01


Mathematics, 23.10.2020 03:01

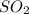 = 64.06 g
= 64.06 g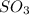 = 80 g/mole
= 80 g/mole