
Chemistry, 27.08.2019 22:20 aaronjin4443
Assume the solubility of benzoic acid in ice-cold water is 1.70 g/l and the solubility of benzoic acid in hot water is 68.0 g/l. calculate the minimum volume of water (in ml) needed to recrystallize 0.900 g of benzoic acid.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3


Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
Assume the solubility of benzoic acid in ice-cold water is 1.70 g/l and the solubility of benzoic ac...
Questions




Computers and Technology, 23.10.2020 20:10

Arts, 23.10.2020 20:10




English, 23.10.2020 20:10

Mathematics, 23.10.2020 20:10

Mathematics, 23.10.2020 20:10

Mathematics, 23.10.2020 20:10



Mathematics, 23.10.2020 20:10



Mathematics, 23.10.2020 20:10

Mathematics, 23.10.2020 20:10

History, 23.10.2020 20:10

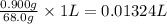 of water.
of water.

