
Chemistry, 28.08.2019 05:10 quaseabrough1
If the solubility product of silver chromate is 2 x 10-12 at 25°c, what is the solubility in mole/liter of silver chromate?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
If the solubility product of silver chromate is 2 x 10-12 at 25°c, what is the solubility in mole/li...
Questions




Mathematics, 23.09.2021 04:30


Mathematics, 23.09.2021 04:30

Mathematics, 23.09.2021 04:30




Mathematics, 23.09.2021 04:30

Mathematics, 23.09.2021 04:30

Mathematics, 23.09.2021 04:30

Geography, 23.09.2021 04:30





Computers and Technology, 23.09.2021 04:30

History, 23.09.2021 04:30

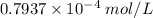
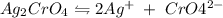
![[Ag^{+}]](/tpl/images/0204/6815/52db7.png) = 2x,
= 2x, ![[CrO4^{2-}]](/tpl/images/0204/6815/1e0e5.png) = x
= x![Ksp=[Ag^{+}]^{2}[CrO_4^{2-}]\\2\times10^{-12}=(2x)^{2}(x)\\2\times10^{-12}=(4x)^{3}\\(x)^{3} = \frac{2\times10^{-12}}{4}\\\\\sqrt[3]{5\times10^{-13}}](/tpl/images/0204/6815/b30f0.png)
![x=\sqrt[3]{0.5\times10^{-12}} =0.7937\times10^{-4}\;mol/L](/tpl/images/0204/6815/ab71d.png)


