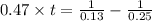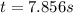Chemistry, 28.08.2019 17:10 abigail251
The rate constant for a particular second-order reaction is 0.47 m-1s-1. if the initial concentration of reactant is 0.25 mol/l, it takes s for the concentration to decrease to 0.13 mol/l.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

You know the right answer?
The rate constant for a particular second-order reaction is 0.47 m-1s-1. if the initial concentratio...
Questions

Geography, 19.10.2019 09:30

Mathematics, 19.10.2019 09:30

Mathematics, 19.10.2019 09:30



English, 19.10.2019 09:30

Mathematics, 19.10.2019 09:30

Mathematics, 19.10.2019 09:30


Mathematics, 19.10.2019 09:30

Mathematics, 19.10.2019 09:30


Mathematics, 19.10.2019 09:30

English, 19.10.2019 09:30


Mathematics, 19.10.2019 09:30




Social Studies, 19.10.2019 09:30

![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0206/1131/ccade.png)
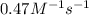
![[A_t]](/tpl/images/0206/1131/5262c.png) = final concentration = 0.13 mole/L = 0.13 M
= final concentration = 0.13 mole/L = 0.13 M![[A_o]](/tpl/images/0206/1131/dc622.png) = initial concentration = 0.25 mole/L = 0.25 M
= initial concentration = 0.25 mole/L = 0.25 M