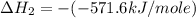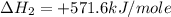Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1
You know the right answer?
Given that δh = −571.6 kj/mol for the reaction 2 h2(g) + o2(g) → 2 h2o(l), calculate δh for these re...
Questions

History, 28.07.2019 23:30


History, 28.07.2019 23:30


History, 28.07.2019 23:30


Spanish, 28.07.2019 23:30


Biology, 28.07.2019 23:30

Health, 28.07.2019 23:30



English, 28.07.2019 23:30

SAT, 28.07.2019 23:30


English, 28.07.2019 23:30

History, 28.07.2019 23:30

English, 28.07.2019 23:30

English, 28.07.2019 23:30

Mathematics, 28.07.2019 23:30

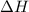 for the reaction is +571.6 kJ/mole.
for the reaction is +571.6 kJ/mole.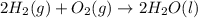
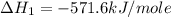
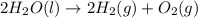
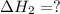
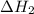 for the reaction will be:
for the reaction will be: