
Determine the number of moles of the compound and determine the number of moles of each type of atom in each of the following. a. 25.0 g of propylene, c3h6b. 3.06 x 10^-3g of the amino acid glycine, c2h5no2c. 25 lb of the herbicide treflan, c13h16n2o4f (1lb = 454 g)d. 0.125 kg of the insecticide paris green, cu4(aso3)2(ch3co2)2e. 325 mg of aspirin, c6h4(co2h)(co2ch3)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
Determine the number of moles of the compound and determine the number of moles of each type of atom...
Questions






English, 09.11.2019 06:31


Biology, 09.11.2019 06:31


Mathematics, 09.11.2019 06:31



Biology, 09.11.2019 06:31

Mathematics, 09.11.2019 06:31

Law, 09.11.2019 06:31


Mathematics, 09.11.2019 06:31



Mathematics, 09.11.2019 06:31

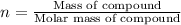
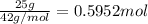
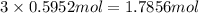 of carbon
of carbon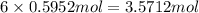 of hydrogen
of hydrogen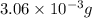
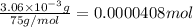
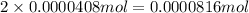 of carbon atom
of carbon atom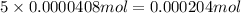 of hydrogen atom
of hydrogen atom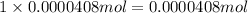 of nitrogen
of nitrogen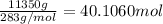
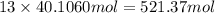 of carbon atom
of carbon atom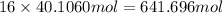 of hydrogen atom
of hydrogen atom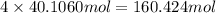 of oxygen atom
of oxygen atom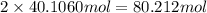 of nitrogen atom
of nitrogen atom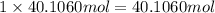 of fluorine
of fluorine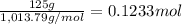
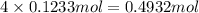 of carbon atom
of carbon atom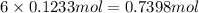 of hydrogen atom
of hydrogen atom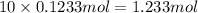 of oxygen atom
of oxygen atom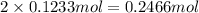 of fluorine
of fluorine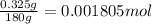
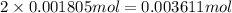 of carbon atom
of carbon atom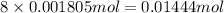 of hydrogen atom
of hydrogen atom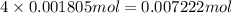 of oxygen atom
of oxygen atom


