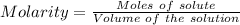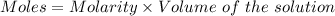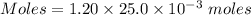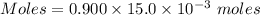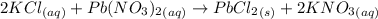Chemistry, 28.08.2019 17:30 leomessifanboy678
A25.0-ml sample of a 1.20 m potassium chloride solution is mixed with 15.0 ml of a 0.900 m lead(ii) nitrate solution and this precipitation reaction occurs: 2 kcl(aq)+pb(no3)2(aq)→pbcl2(s)+2 kno3(aq) the solid pbcl2 is collected, dried, and found to have a mass of 2.45 g. determine the limiting reactant, the theoretical yield, and the percent yield.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3


You know the right answer?
A25.0-ml sample of a 1.20 m potassium chloride solution is mixed with 15.0 ml of a 0.900 m lead(ii)...
Questions


Social Studies, 06.05.2020 02:38




Mathematics, 06.05.2020 02:38

Mathematics, 06.05.2020 02:38



Mathematics, 06.05.2020 02:38

Mathematics, 06.05.2020 02:38


Mathematics, 06.05.2020 02:38

Mathematics, 06.05.2020 02:38

Mathematics, 06.05.2020 02:38



Mathematics, 06.05.2020 02:38

Mathematics, 06.05.2020 02:38