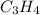Chemistry, 28.08.2019 21:10 heavenwagner
Cumene is a compound containing only carbon and hydrogen that is used in the production of acetone and phenol in the chemical industry. combustion of 47.6 mg cumene produces some co2 and 42.8 mg water. the molar mass of cumene is between 115 and 125 g/mol. determine the empirical and molecular formulas.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3


Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
Cumene is a compound containing only carbon and hydrogen that is used in the production of acetone a...
Questions

Mathematics, 26.05.2021 06:20

Biology, 26.05.2021 06:20


Mathematics, 26.05.2021 06:20


Mathematics, 26.05.2021 06:20

Mathematics, 26.05.2021 06:20



English, 26.05.2021 06:20

Health, 26.05.2021 06:20




English, 26.05.2021 06:20

Mathematics, 26.05.2021 06:20




Biology, 26.05.2021 06:20

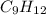
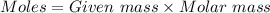
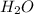 = 42.8/18 = 2.3778×10⁻³ moles
= 42.8/18 = 2.3778×10⁻³ moles