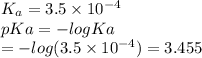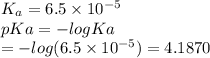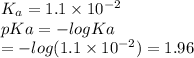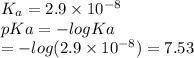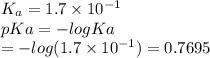Chemistry, 28.08.2019 21:30 Andrewecolt1993
Which of the following acids (listed with ka values) and their conjugate base would form a buffer with a ph of 2.34? a. hf, ka = 3.5 x 10-4 b. c6h5cooh, ka = 6.5 x 10-5 c. hclo2, ka = 1.1 x 10-2 d. hclo, ka = 2.9 x 10-8 e. hio3, ka = 1.7 x 10-1

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
Which of the following acids (listed with ka values) and their conjugate base would form a buffer wi...
Questions


English, 19.07.2019 18:30

History, 19.07.2019 18:30


Mathematics, 19.07.2019 18:30




Mathematics, 19.07.2019 18:30

Spanish, 19.07.2019 18:30

History, 19.07.2019 18:30







Mathematics, 19.07.2019 18:30

Biology, 19.07.2019 18:30