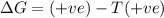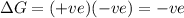For the reaction mgso3(s) → mgo(s) + so2(g), which is spontaneous only at high temperatures, one would predict thata. δh˚ is negative and δs˚ is negativeb. δh˚ is positive and b. δs˚ is negativec. δh˚ is positive and δs˚ is positived. δh˚ is negative and δs˚ is positive

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
You know the right answer?
For the reaction mgso3(s) → mgo(s) + so2(g), which is spontaneous only at high temperatures, one wou...
Questions

Advanced Placement (AP), 22.10.2020 17:01







Mathematics, 22.10.2020 17:01


Health, 22.10.2020 17:01


Mathematics, 22.10.2020 17:01


History, 22.10.2020 17:01

Mathematics, 22.10.2020 17:01


Mathematics, 22.10.2020 17:01


Mathematics, 22.10.2020 17:01

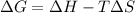
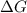 = Gibbs free energy
= Gibbs free energy 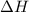 = enthalpy change
= enthalpy change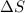 = entropy change
= entropy change 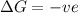
 >
> 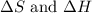 both have positive values.
both have positive values.