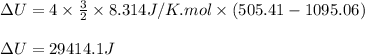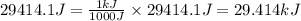Chemistry, 28.08.2019 23:00 michaellangley
Acompression, at a constant pressure of 140 kpa, is performed on 4.0 moles of an ideal monatomic gas (cv = 3/2 r). the compression reduces the volume of the gas from 0.26 m^3 to 0.12 m^3. the change in the internal energy of the gas, in kj is ("^3" means to the power of 3)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1
You know the right answer?
Acompression, at a constant pressure of 140 kpa, is performed on 4.0 moles of an ideal monatomic gas...
Questions


Health, 07.07.2019 10:00

Mathematics, 07.07.2019 10:00

Mathematics, 07.07.2019 10:00



Physics, 07.07.2019 10:00

World Languages, 07.07.2019 10:00

English, 07.07.2019 10:00



Mathematics, 07.07.2019 10:00


History, 07.07.2019 10:00

Advanced Placement (AP), 07.07.2019 10:00




Mathematics, 07.07.2019 10:00

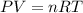
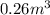
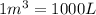
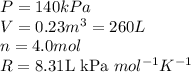
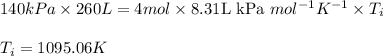
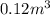
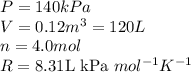
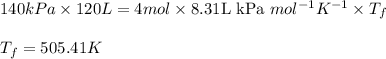
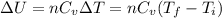
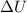 = change in internal energy = ?
= change in internal energy = ?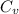 = heat capacity at constant volume =
= heat capacity at constant volume = 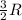
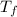 = final temperature = 1095.06 K
= final temperature = 1095.06 K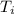 = initial temperature = 505.41 K
= initial temperature = 505.41 K