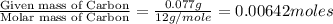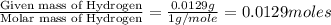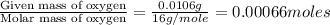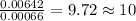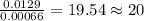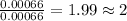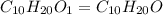Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
Menthol, the substance we can smell in mentholated cough drops, is composed of carbon, hydrogen, and...
Questions



History, 13.04.2020 20:41

Mathematics, 13.04.2020 20:41


History, 13.04.2020 20:41


Mathematics, 13.04.2020 20:41


Mathematics, 13.04.2020 20:41

Mathematics, 13.04.2020 20:41



Health, 13.04.2020 20:41

Mathematics, 13.04.2020 20:41




Mathematics, 13.04.2020 20:42

Mathematics, 13.04.2020 20:42

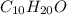
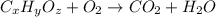
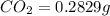
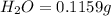
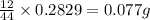 of carbon will be contained.
of carbon will be contained.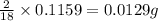 of hydrogen will be contained.
of hydrogen will be contained.