
Chemistry, 29.08.2019 01:20 emmapizano
Use the following, balanced equation for the question below: 2al(s) + 3cucl2(aq) → 3cu(s) + 2alcl3(aq) how many moles of copper metal will be produced from 10.0 moles of aluminum metal? 10.0 moles of copper 15.0 moles of copper 20.0 moles of copper 30.0 moles of copper

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
Use the following, balanced equation for the question below: 2al(s) + 3cucl2(aq) → 3cu(s) + 2alcl3(...
Questions



Mathematics, 07.12.2020 17:00


Spanish, 07.12.2020 17:00

Mathematics, 07.12.2020 17:00

Mathematics, 07.12.2020 17:00



Spanish, 07.12.2020 17:00





English, 07.12.2020 17:00

Mathematics, 07.12.2020 17:00

Mathematics, 07.12.2020 17:00


Business, 07.12.2020 17:00

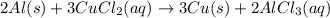
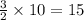 moles of copper.
moles of copper.

