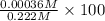Chemistry, 29.08.2019 18:00 lhmsokol56
Calculate the percent ionization of nitrous acid in a solution that is 0.222 m in nitrous acid (hno3) and 0.278 m in potassium nitrite (kno2). the acid dissociation constant of nitrous acid is 4.50 x 10-4 a) 55.6 b) 0.162 c) 15.5 d) 2.78 * 10-3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3


Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Calculate the percent ionization of nitrous acid in a solution that is 0.222 m in nitrous acid (hno3...
Questions

Mathematics, 11.12.2021 01:00




Mathematics, 11.12.2021 01:00



Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00


History, 11.12.2021 01:00







Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00

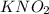 is 0.278 M and it is completely ionized into
is 0.278 M and it is completely ionized into 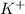 and
and 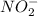 .
.![[KNO_{2}]](/tpl/images/0209/3896/fb191.png) =
= ![[NO_{2}]](/tpl/images/0209/3896/53e25.png) = 0.278 M
= 0.278 M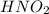 is 0.222 M.
is 0.222 M.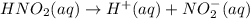
![K_{a} = \frac{[H^{+}][NO^{-}_{2}]}{[HNO_{2}]}](/tpl/images/0209/3896/218b7.png)
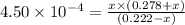
![[H^{+}]](/tpl/images/0209/3896/85507.png) is 0.00036 M. Therefore, percentage ionization of
is 0.00036 M. Therefore, percentage ionization of ![\frac{[H^{+}]}{[HNO_{2}]} \times 100](/tpl/images/0209/3896/26d17.png)