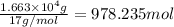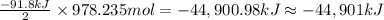Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
The production of ammonia (nh3) under standard conditions at 25°c is represented by the following th...
Questions










English, 12.01.2021 20:20


Mathematics, 12.01.2021 20:20

Arts, 12.01.2021 20:20

Mathematics, 12.01.2021 20:20


Mathematics, 12.01.2021 20:20

Mathematics, 12.01.2021 20:20

Physics, 12.01.2021 20:20

Mathematics, 12.01.2021 20:20

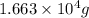 grams of ammonia is produced.
grams of ammonia is produced.