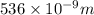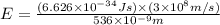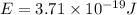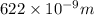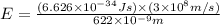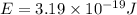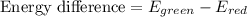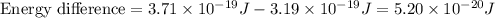Chemistry, 29.08.2019 20:00 emblemhacks
enter your answer in the provided box.
how much more energy per photon is there in green light of wavelength 536 nm than in red light of wavelength
622 nm?
__× 10 __ j
(enter your answer in scientific notation.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1

Chemistry, 23.06.2019 09:30
How many moles of na2s2o3 are needed to react with 0.12mol of cl2? show work.
Answers: 1

Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3
You know the right answer?
enter your answer in the provided box.
how much more energy per photon is there in green light...
how much more energy per photon is there in green light...
Questions



Social Studies, 29.01.2020 22:58

Mathematics, 29.01.2020 22:58

Mathematics, 29.01.2020 22:58

Mathematics, 29.01.2020 22:58


World Languages, 29.01.2020 22:58

Mathematics, 29.01.2020 22:58


Mathematics, 29.01.2020 22:58

Mathematics, 29.01.2020 22:58

Mathematics, 29.01.2020 22:58

History, 29.01.2020 22:58

Biology, 29.01.2020 22:58

Biology, 29.01.2020 22:58

English, 29.01.2020 22:58

Computers and Technology, 29.01.2020 22:58

Mathematics, 29.01.2020 22:58

Mathematics, 29.01.2020 22:58

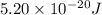
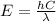
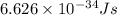
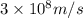
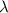 = wavelength of light = 536 nm =
= wavelength of light = 536 nm =