

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
Asolution is made initially with 0.200 m hio3 (kc = 0.17). once the equilibrium below is established...
Questions

Computers and Technology, 18.08.2021 14:00

Mathematics, 18.08.2021 14:00


English, 18.08.2021 14:00


Chemistry, 18.08.2021 14:00

Business, 18.08.2021 14:00

Mathematics, 18.08.2021 14:00

History, 18.08.2021 14:00

Mathematics, 18.08.2021 14:00

Mathematics, 18.08.2021 14:00

Mathematics, 18.08.2021 14:00


Social Studies, 18.08.2021 14:00

Mathematics, 18.08.2021 14:00

Biology, 18.08.2021 14:00

Biology, 18.08.2021 14:00

Geography, 18.08.2021 14:00

Mathematics, 18.08.2021 14:00

Biology, 18.08.2021 14:00



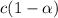

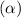 .
.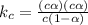
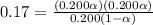
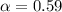
![[H^+]=c\alpha=0.200\times 0.59=0.118M](/tpl/images/0210/0415/e6cb2.png)



