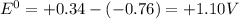Chemistry, 30.08.2019 00:30 mixcolin0002
it is expected that a chemical reaction will occur when copper metal is combined with aqueous zinc sulfate. explain why there will be no reaction when zinc metal and aqueous copper sulfate solution are combined. identify the anode and the cathode, assuming a voltaic cell is constructed. note: be careful in the calculation of the standard cell potential ( eo cathode - eo anode). do not change the sign of the given reduction potential. the sign is already taken care of using the formula for calculating the standard cell potential.
standard reduction potential: cu2+(aq) + 2e- > cu(s) eo = -0.34 v
zn2+(aq) + 2e- > zn(s) eo = -0.76 v
standard cell potential = eo cathode - eo anode
although , combination of the reactants do not result in voltaic cells, it can be predicted if the reaction is spontaneous, based on the standard reduction potential.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
You know the right answer?
it is expected that a chemical reaction will occur when copper metal is combined with aqueous zinc s...
Questions


Mathematics, 25.03.2021 05:40


Chemistry, 25.03.2021 05:40


Chemistry, 25.03.2021 05:40


Mathematics, 25.03.2021 05:40

Health, 25.03.2021 05:40


Chemistry, 25.03.2021 05:40


Mathematics, 25.03.2021 05:40

Mathematics, 25.03.2021 05:40

Mathematics, 25.03.2021 05:40

Mathematics, 25.03.2021 05:40

Mathematics, 25.03.2021 05:40


Mathematics, 25.03.2021 05:40

 = +ve, reaction is spontaneous
= +ve, reaction is spontaneous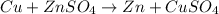
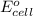 = standard electrode potential =
= standard electrode potential =
 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Cu^{2+}/Cu]}= +0.34V](/tpl/images/0210/3859/55543.png)
![E^0_{[Zn^{2+}/Zn]}= -0.76V](/tpl/images/0210/3859/6c8c3.png)
![E^0=E^0_{[Zn^{2+}/Zn]}- E^0_{[Cu^{2+}/Cu]}](/tpl/images/0210/3859/f1898.png)

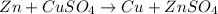
![E^0=E^0_{[Cu^{2+}/Cu]}- E^0_{[Zn^{2+}/Zn]}](/tpl/images/0210/3859/cb03b.png)