
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these are decomposed, the sulfur dioxide produces 3.46 g oxygen and 3.47 g sulfur, while the sulfur trioxide produces 8.25 g oxygen and 5.50 g sulfur. a) calculate the mass of oxygen per gram of sulfur for sulfur dioxide.
b) calculate the mass of oxygen per gram of sulfur for sulfur trioxide.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these are decomposed...
Questions

Health, 01.09.2019 18:50


Mathematics, 01.09.2019 18:50

Geography, 01.09.2019 18:50

Mathematics, 01.09.2019 18:50

Biology, 01.09.2019 18:50

Computers and Technology, 01.09.2019 18:50

Physics, 01.09.2019 18:50




Chemistry, 01.09.2019 18:50

History, 01.09.2019 18:50

Biology, 01.09.2019 18:50

English, 01.09.2019 18:50

Mathematics, 01.09.2019 18:50


History, 01.09.2019 18:50


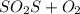
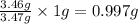 of oxygen will form.
of oxygen will form.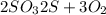
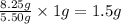 of oxygen will form.
of oxygen will form.

