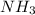Chemistry, 30.08.2019 01:30 natjojo0512
Consider the following balanced reaction between hydrogen and nitrogen to form ammonia: 3h2(g) + n2(g)→2nh3(g)how many moles of nh3 can be produced from 24.0 mol of h2 and excess n2? express the number of moles to three significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
Consider the following balanced reaction between hydrogen and nitrogen to form ammonia: 3h2(g) + n2(...
Questions

Mathematics, 15.07.2021 01:00


Mathematics, 15.07.2021 01:00

Mathematics, 15.07.2021 01:00


Computers and Technology, 15.07.2021 01:00



Social Studies, 15.07.2021 01:00


Mathematics, 15.07.2021 01:00






Mathematics, 15.07.2021 01:00

Mathematics, 15.07.2021 01:00

Biology, 15.07.2021 01:00

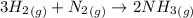
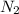 is present in large excess. It means that
is present in large excess. It means that 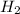 is the limiting reagent and the formation of the product is governed by
is the limiting reagent and the formation of the product is governed by